

|
|
Clin. Cardiol. Vol. 23 (Suppl. I), I-4–I-7 (2000)
Alexander G.G. Turpie, M.B., F.R.C.P. (Lond., Glasg.), F.A.C.P., F.A.C.C., F.R.C.P.C.
Department of Medicine, Hamilton Health Sciences Corporation, General Division, Hamilton, Ontario, Canada
Summary: The low-molecular-weight heparins (LMWHs) have a number of therapeutic advantages, relative to standard unfractionated heparin (UFH). They are readily bioavailable when injected subcutaneously and can be given in fixed doses, allowing for far simpler administration.
Several LMWHs are now commercially available, each demonstrating different physical and chemical properties and different activities in animal models of anticoagulation or hemorrhage. In clinical comparisons with placebo in the treatment of unstable coronary artery disease (UCAD), the LMWHs dalteparin sodium and nadroparin calcium have demonstrated good anticoagulant efficacy. In comparisons with UFH, on the other hand, only enoxaparin has shown superior anticoagulant activity, as reported in the results of the Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-wave Coronary Events (ESSENCE) and Thrombolysis In Myocardial Infarction (TIMI) 11B trials. However, close scrutiny of the methodology of the clinical trials in UCAD reveals considerable differences in study designs, dosage regimens, duration of administration of active treatments, and the timing and definition of endpoints. Therefore, it would not be scientifically sound to compare results with the different LMWHs based on the current available studies. It is also not possible to draw any conclusions with regard to the relative efficacy of the different LMWHs, since there are no properly-sized comparative data between dalteparin sodium, enoxaparin sodium, and nadroparin calcium.
Key words: unstable coronary artery disease, low-molecular-weight heparins, anticoagulation, dalteparin sodium, enoxaparin sodium, nadroparin calcium
Unfractionated heparin (UFH) has, for some time, been established in the management of deep vein thrombosis (DVT) and in thromboprophylactic indications. In addition, UFH plays an important role in the primary and secondary prevention of acute coronary syndromes (ACSs), in maintaining coronary artery patency in patients with myocardial ischemia treated with thrombolytic agents, and in preventing the recurrence of embolic stroke.1
In spite of its wide acceptance and application, UFH is associated with a number of limitations, including a short duration of action, poor bioavailability after subcutaneous injection, an unpredictable anticoagulant response, a risk of heparin-induced thrombocytopenia (HIT),2 and the problem of disease reactivation following the withdrawal of treatment.3,4 Attempts to overcome these problems have been directed principally toward modification of UFH to produce new antithrombotic agents, and this has resulted in the development and introduction of the low-molecular-weight heparins (LMWHs). The following discussions will examine whether it is possible to differentiate between three of the most widely used LMWHs in ACS: dalteparin sodium, enoxaparin sodium, and nadroparin calcium.
Unfractionated heparin is a mixture of glycosoaminoglycan polymers with an average molecular weight of 15 kDa (range 5–30 kDa). Chemical or enzymatic depolymerization of UFH yields the LMWHs, which have a mean molecular weight of around 5 kDa. All of these molecules possess a specific pentasaccharide moiety that inhibits activated coagulation factors and thus prevents clotting. In addition, the larger polymers are capable of binding both antithrombin and Factor IIa.
It has been shown that Factor IIa inhibition is determined by the number of monosaccharide units contained within heparin polymers. Compounds with 5 to 18 monosaccharide units inhibit only Factor Xa, whereas those with 18 to 26 monosaccharides will inhibit both Factor Xa and Factor IIa.5 Since the larger molecules that are necessary for the inhibition of Factor IIa are present in lower numbers in the lower-mass fragments than in UFH, the LMWHs are less potent inhibitors of Factor IIa and thus affect the clotting time to a lesser degree than UFH.
Although the depolymerization methods used to prepare the LMWHs from UFH result in products with similar properties, structural variations exist among the molecules and influence their respective biological activities (Fig. 1).6,7 The counter-ions associated with these products also vary: while nadroparin is a calcium salt, the other LMWHs are all sodium salts. Further differences relate to the mean molecular weights, saccharide chain length distributions, ability to inhibit tissue factor pathway inhibitor (TFPI), and susceptibility to inhibition by Platelet Factor 4.8 All of these factors may impart unique properties to each LMWH and may give rise to variations in response in vivo.
In contrast to UFH, the LMWHs do not bind to plasma proteins. This characteristic is an important contributory factor to the approximately 3- to 4-fold greater bioavailability of the LMWHs following subcutaneous (SC) administration, relative to UFH,7 and enables the fixed-dose, SC administration that is such an important advantage in clinical use.
The anticoagulant potency of standard UFH is usually measured in terms of USP (US Pharmacopoeia) U/kg. The relative anticoagulant potency of the LMWHs is lower than that of standard UFH (40–75 U/mg for the LMWHs, compared with 150 U/mg for UFH). Using a rabbit stasis thrombosis model, the antithrombotic activity of LMWHs has been measured as anti-Xa activity. The median effective antithrombotic dose of nadroparin and enoxaparin was found to be more than three times higher than that of UFH, while the median effective dose of dalteparin sodium was nearer to twice that of UFH.7 In animal models of hemorrhage, heparins with higher molecular weights exhibit greater hemorrhagic potential following intravenous (IV) administration than those with lower molecular weights.7 These data suggest that anti-Xa dose adjustment may not result in therapeutic equivalence of these agents, in terms of antithrombotic efficacy, following IV administration.
Bleeding is the major complication associated with the use of UFH. In cases of serious bleeding, protamine sulphate can be administered to neutralize UFH, but there has been some concern that protamine may not adequately neutralize the LMWHs. When the degree of protamine neutralization was assessed by assaying anti-Xa and anti-IIa activities before and after supplementation of dalteparin sodium, enoxaparin sodium, and nadroparin calcium with protamine, the anti-Xa activity neutralization was found to be only in the region of 30–45%. With UFH, on the other hand, almost 100% neutralization was achieved. Factor IIa activity, by contrast, was completely neutralized in both the LMWHs and in standard UFH.7
Heparin-induced thrombocytopenia (HIT) is another well-recognized complication of UFH treatment. Two types of HIT are recognized clinically, namely a mild thrombocytopenia of early onset and a severe, delayed-onset thrombocytopenia, which is caused by an immune mechanism.9 While there have been reports of LMWHs being used successfully as alternatives to UFH in patients requiring early management for the manifestations of HIT,10 the level of cross-reactivity of the LMWHs with heparin-induced antibody is still such that it would preclude the administration of LMWHs to patients in whom heparin-induced antibodies have already caused thrombocytopenia, but who still require anticoagulation. For such individuals, the heparinoid, danaparoid sulphate or the direct thrombin inhibitor, hirudin, would be more appropriate therapeutic agents.
Given the demonstrable biological and chemical differences that exist between the LMWHs, it may be expected that these would translate into variations in clinical activity. Plasma clearance characteristics are known to vary among these compounds, giving rise to differences in dosage regimens; however, the question of whether the preclinical differences between the LMWHs also translate into differences in clinical efficacy and associated bleeding complications has yet to be answered.
Clinical comparisons have been carried out of the LMWHs versus UFH in the management of patients with unstable angina (UA) or non-Q-wave myocardial infarction (NQMI). Nadroparin calcium was evaluated in the FRAXiparine in Ischaemic Syndromes (FRAX.I.S) study,11 dalteparin sodium in the FRagmin In unstable Coronary artery disease (FRIC) study,12 and enoxaparin sodium in the Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-wave Coronary Events (ESSENCE)13 and in the Thrombolysis In Myocardial Infarction (TIMI) 11B studies.14 While both dalteparin sodium and nadroparin calcium had previously been found to demonstrate greater efficacy than placebo in the management of UA and NQMI, the FRAX.I.S and FRIC studies showed that clinical outcomes with these products are similar to those obtained with standard UFH. Enoxaparin sodium, however, was found to yield greater clinical outcome benefits than UFH in these indications in both the ESSENCE and the TIMI 11B trials.15
So, how can these apparent differences in clinical efficacy be explained? It is possible that the differences in the pharmacokinetic profiles of the agents could be a contributory factor. Of the three molecules, dalteparin sodium has the largest mean molecular weight (5.7 kDa), while nadroparin calcium is somewhat smaller (4.5 kDa), and enoxaparin sodium is the smallest of the three (4.4 kDa).6 These differences are reflected in the different elimination half-lives of 2.8, 3.7, and 4.4 h for dalteparin sodium, nadroparin calcium, and enoxaparin sodium, respectively.16
Besides the intrinsic differences that exist between the molecules themselves, variations in the design and methodology of the clinical trials must also be taken into consideration. A summary of the trial designs is presented in Table I, from which it can be seen that there were significant differences in aspects such as patient selection, relative doses of medication, active treatment duration, and the definition and assessment of endpoints. Of the four trials, FRIC was the only open study, all the others being performed blinded. In the FRIC study, patients were recruited up to 72 h after symptom onset, compared with a cut-off time of 24 h after the last episode of chest pain in the ESSENCE and TIMI 11B trials. Thus, the FRIC trial patient population may have been less acutely ill. It is of importance that, although all these trials compared the LMWHs with UFH, the dosage and duration of UFH treatment varied so widely that not even a direct comparison of the control group data is possible (Table I). Patients randomized to UFH received 5 to 6 days of active treatment in the FRAX.I.S and FRIC trials, but only 2 to 3 days of treatment in ESSENCE and TIMI 11B. Since several days may be required for a good APTT to be established with UFH, it is possible that the trial outcomes may have been significantly influenced by these variations in treatment duration. It has been shown that an average of 5 to 6 days' treatment with UFH plus aspirin reduces the incidence of death or myocardial infarction (MI) relative to aspirin alone,17,18 whereas no such reduction is seen when the duration of UFH treatment is reduced to 2 days.19
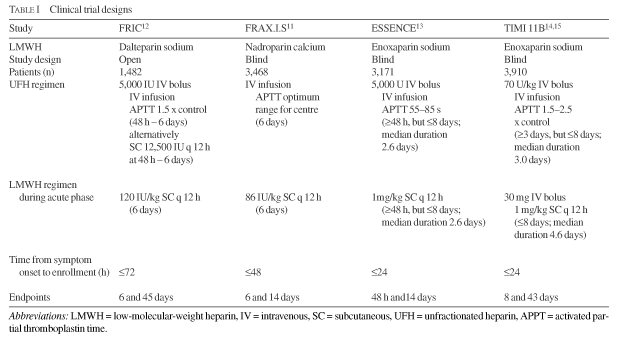
The baseline characteristics of the patient populations in the clinical trials are summarized in Table II. The percentages of patients with a previous MI or NQMI were higher in the ESSENCE and TIMI 11B studies than in FRAX.I.S and FRIC. The enoxaparin trials also included patients who were using greater quantities of aspirin at recruitment.

Although not a primary endpoint, the incidence of recurrent angina was assessed in all the studies comparing LMWHs with UFH. However, once again direct comparisons between studies are not possible, in this case because of differences in the definitions of recurrent angina. In the FRIC study, recurrent angina was defined as chest pain requiring an IV nitroglycerin infusion. In the ESSENCE and TIMI 11B trials, the same condition was defined as (1) rest angina of at least 5-min duration, associated with either a new ST-segment shift or a T-wave inversion on electrocardiography; or (2) angina that prompted revascularization; or (3) postdischarge angina that prompted rehospitalization.
Thus, the number and nature of the differences among the FRIC, FRAX.I.S, ESSENCE, and TIMI 11B study designs (Table I) could explain why differences were observed in the composite outcomes for patients randomized to UFH treatment in these trials. These differences also mean that any conclusions regarding the relative efficacies of the LMWHs in the treatment of UCAD, reached on the basis of these results, should be treated with considerable caution.
It can be concluded that the LMWHs are different products with different antithrombotic activities in model systems. It can also be stated that use of a LMWH rather than UFH simplifies the management of patients with UCAD, offering easier administration and a reduced risk of complications, and obviating the need for plasma monitoring.
However, it is currently not possible to draw any conclusions with regard to relative clinical efficacy of the different LMWHs. This is true not only because there is an absence of comparative studies of dalteparin sodium, enoxaparin sodium and nadroparin calcium, but also because each of the LMWHs has been evaluated in studies in different patient populations with different study designs, endpoints, and endpoint definitions. This follows also for the comparison of the LMWHs with UFH: again, the comparative studies have been designed differently, using for example differing endpoints and differing UFH treatment durations (UFH was administered for only 2 to 3 days in ESSENCE and TIMI-11B, but for 5 to 6 days in FRIC and FRAX.I.S). Therefore, it would not be scientifically sound to compare results with the different LMWHs versus UFH based on the currently available studies.
References
Address for reprints:
Dr. A.G.G. Turpie
Dept. of Medicine, HGH McMaster Clinic
HHSC-General Division
231 Barton Street East
Hamilton, Ontario, Canada
e-mail: turpiea@fhs.mcmaster.ca